|
PALEOECOLOGY AND BIOFACIES |
ABYSSAL BIOFACIESAlthough Paleogene agglutinated assemblages are very widespread throughout the Atlantic and Tethys, the truly abyssal faunas are limited in their occurrence. Paleogene abyssal assemblages have only been recorded from two localities so far: the Iberian Abyssal Plain and the Celebes Sea (Kaminski & Huang, 1991; Kuhnt & Collins, 1996; Kuhnt & Urquhart, 2001). The backtracked depths for these sites are all below 4,000 metres, and the depth of the CCD in the Atlantic at the time was close to 3,500 m (Tucholke & Vogt, 1979). The assemblages recovered at ODP Site 647 contain elements of an abyssal fauna, but this site was situated above the CCD for much of its history. There are several possible reasons why the Paleogene abyssal faunas were poorly developed: (1) Stable isotopic data indicate the late Paleocene ocean was very productive (Corfield et al., 1991), and that low oxygen conditions occurred at least locally in the deep ocean (Kaiho, 1991). Paleocene sediments deposited below the CCD are greenish or dark grey in most areas of the western Tethys; (2) Most Paleogene sites are located close to the continental margins where terrigenous influx was high; (3) Many Eocene sites in North Atlantic contain cherts, a lithology that is unfavourable for the preservation of foraminifera. (4) At sites were Paleogene claystones were recovered, the agglutinated assemblages are depauperate or not preserved at all (see discussion below). The Iberian Abyssal Plain and the Celebes Sea therefore provide exceptional insight into the record of Paleogene abyssal DWAF. Studies on modern abyssal foraminifera have reached a consensus that both the occurrence and abundance of taxa is closely tied to the trophic continuum (van der Zwaan et al., 1999; Altenbach et al., 1999). In general, assemblages dominated by modern abyssal agglutinated foraminifera represent the most oligotrophic end of the spectrum, inhabiting areas where sea-floor organic flux is below ca. 2.5 gC/m2-yr (Altenbach et al., 1999; De Rijk et al., 2000). Oceanic productivity decreased globally across the Paleocene-Eocene transition (e.g., Corfield & Shackleton, 1988; Thomas & Shackleton, 1996) which brought about the oligotrophic conditions that favour the diversification of abyssal agglutinated foraminifera (Kaminski et al., 1999). Lower Eocene red shales are found throughout the western Tethys, suggesting well-oxygenated conditions and low organic flux in the deep sea (Kaminski et al., 1996). Off Iberia, the Paleogene abyssal assemblages were described by Kuhnt & Collins (1996) and Kuhnt & Urquhart (2001). The Iberian Abyssal Plain was sheltered from terrigenous sedimentation, sediment accumulation rates were generally low, and brownish-red claystones were deposited beneath the CCD. Paleocene assemblages from ODP Sites 1068, 1069 and 1070 contain over 50 species of agglutinated foraminifera and are taxonomically identical to the Alpine-Carpathian faunas. As in other areas, there is a significant reduction in diversity across the Paleocene/Eocene boundary. Lower Eocene sediments recovered at Sites, 897, 900, and 1067-1070 contain a Glomospira assemblage that is similar to the mid-Cretaceous "Biofacies B" assemblage described by Kuhnt & Kaminski (1989) and Kaminski et al., (1992). This distinctive horizon has been found in the western Tethys and northern Atlantic from Poland to Labrador (Kaminski et al., 1989). Above this horizon, the middle to upper Eocene assemblages from reddish-brown clays contain several new species that are not observed in older sediments. The most common new form in the Eocene at the studied localities is Reticulophragmium amplectens. The polyphyletic genus Reticulophragmium evolved independently from several ancestors during the Paleocene and Eocene, and diversified into several lineages (Berggren & Kaminski, 1990). Most of the diverse Reticulophragmium species are observed in high latitudes, while only the diminutive R. amplectens is common in the abyssal Eocene assemblages. Another important Paleogene abyssal species is Haplophragmoides walteri, the supposed ancestor of some species of Reticulophragmium. At least two new species of Karrerulina appeared in the Eocene (K. horrida and K. coniformis). The genus Karrerulina is common today on the Atlantic abyssal plains (Schröder, 1986; Kuhnt et al., 2000), and it displays abundance maxima at several Eocene abyssal localities. In addition, two new species of Pseudonodosinella (P. nodulosa and P. elongata), and one new Ammodiscus (A. latus) appeared during the Eocene. A second very diminutive species of Ammodiscus (A. nagyi) is found at Site 647 in the Labrador Sea and at the deepest sites in the Norwegian Sea. Alongside these forms are the ubiquitous tubular forms, which generally dominate the assemblages. Abyssal varieties are very small, finely branching forms such as Rhizammina, Bathysiphon, or the delicate species Hyperammina kenmilleri Kaminski, 1989. In the Indo-Pacific region, middle Eocene to early Oligocene deep-waters were well oxygenated, and pelagic red clays were deposited in some areas. The only site where DWAF were recovered from abyssal red clays of Eocene-Oligocene age was at ODP Site 767 in the Celebes Sea (Kaminski & Huang, 1991). In addition to the tubular forms, the middle to upper Eocene red clays contain common Reticulophragmium amplectens. The abyssal agglutinated facies persisted into the Oligocene at this locality, and is characterised by a low diversity assemblage with Reticulophragmium acutidorsatum and Pseudonodosinella elongata. In general, the abyssal Paleogene DWAF faunas from the Iberian Abyssal Plain and Celebes Sea ODP sites display lower diversity than the coeval Western Tethyan and Carpathian flysch-type and slope assemblages. In all the areas studied, the diversity and abundance of DWAF declines in the mid-late Eocene. A similar trend is observed among the abyssal calcareous benthic foraminifera (Berggren & Olsson, 1985). By the Oligocene, the remaining genera at Site 767 consist of forms with wide stratigraphical and bathymetrical ranges. An important finding is that no Eocene-Oligocene species found up to now is unique to the abyssal plain - all are also known to occur in the flysch-type faunas (see below). This is in stark contrast with the Late Cretaceous "Krashenninikov fauna" (Kaminski et al., 1999) which contained several unique species of Haplophragmoides and Paratrochamminoides. Miocene abyssal sediments studied by Chen et al., (1997) from piston cores collected in the Central Pacific contain very depauperate assemblages consisting mainly of Pseudonodosinella nodulosa and Psamminopelta gradsteini.
The type localities for the Late Cretaceous abyssal biofacies known as the "Krashenninikov Fauna", are the abyssal plains of the North Pacific and Indian Oceans. During the Cretaceous these areas were located beneath the subtropical oceanic gyres, far from land. The North Pacific Gyre is today the most oligotrophic region of the oceans. We have observed elements of the Krashenninikov fauna in the Upper Cretaceous Plantagenet Formation from Sites 137, where it is best developed, as well as at Sites 543, 603, and 641. Jansa et al. (1979) interpreted the Plantagenet Formation as having been deposited in an oxygenated, pelagic deep-sea environment similar to the environment presently accumulating pelagic clays in the central North Pacific. However, by Paleocene time the trophic continuum changed, and assemblages present at Site 543 in the equatorial Atlantic and at ODP sites on the Iberian Abyssal Plain are depauperate.
|
THE "FLYSCH-TYPE" AGGLUTINATED FORAMINIFERAL BIOFACIESThe flysch-type biofacies is known from most of the studied localities, and was originally described by Gradstein & Berggren (1981). It typically characterises the clastic substrates, and in most areas is found within the hemipelagic intercalations of the turbidic flysch-sequences. The term refers to the fact that these assemblages are found in either tectonically active flysch basins such as the Alpine-Carpathian basins, or in the rapidly subsiding troughs that are found around the margins of the continents, in areas such as the North Sea and Viking Graben. In the flysch-type assemblages, tubular agglutinated forms such as Bathysiphon, Nothia, Rhabdammina, and Rhizammina are often the dominant component of the assemblages, which led Brouwer (1965) to refer to them as "Rhabdammina faunas". In the North Atlantic area, we observe the flysch-type assemblages in various palaeogeographical settings, from bathyal slope environments to distal turbiditic environments at the base of the continental rise, to areas influenced by contour currents. The Carpathian Upper Cretaceous to Paleogene assemblages reported by the early workers all described what we term flysch-type assemblages. Many of the cosmopolitan taxa of DWAF were first described from the turbiditic Godula, Ropianka (= Inoceramian beds), Istebna, and Hieroglyphic beds of Poland, Romania, and the Czech Republic. However, despite 100 years of investigation, there are still few quantitative studies from the area (it was not routine practise to count samples quantitatively). Only the more recent studies of Bubík (1995) and Bąk (2004) provide quantitative foraminiferal data from Carpathian flysch units. The Flysch-type assemblages are known from the tectonically active Atlantic margins, including southern Trinidad, the Moroccan Rif, and also from the sub-Betic units of southern Spain. The younger Cretaceous and Palaeogene assemblages from these areas have been described by Kuhnt (1987), Kaminski et al., (1988); Kuhnt & Kaminski (1989), and Kaminski et al., (1996). Four DSDP/ODP Sites in the eastern Atlantic have typical flysch-type assemblages: At Site 367, 368, and 141, this biofacies occurs in Upper Cretaceous reddish silty claystones. The assemblages from Hole 367 were initially studied by Krashenninikov & Pflaumann (1977). At ODP Site 959, the flysch-type assemblages continue upward into Paleocene greenish claystones (Kuhnt et al., 1998).
The North Atlantic flysch-type biofacies contains the most diversified assemblages encountered. Most characteristic for this biofacies is the occurrence of coarsely agglutinated forms, such as some species of Ammobaculites, Psammosphaera, Saccammina, and thick-walled tubular forms such as Nothia and Psammosiphonella. Some genera appear to be almost restricted to the flysch-type biofacies, such as Rzehakina, coarsely-agglutinated ammodiscids, and diverse species of Paratrochamminoides and Trochamminoides. Large-sized taxa, such as Hyperammina dilatata, Hormosina velascoensis, Placentammina placenta, Trochamminoides grzybowskii, and T. proteus are also characteristic of this biofacies. However, as much as the presence of coarsely-agglutinated forms is characteristic of the flysch-type assemblage, the absence of some other species, such as the "Krashenninikov species" and the fragile abyssal agglutinates of the Scaglia biofacies (see below) is an equally important feature of this biofacies.
|
THE SCAGLIA-TYPE AGGLUTINATED FORAMINIFERAL BIOFACIESOn the submerged continental fragments of the Western Tethys, a distinctive assemblage of small-sized agglutinated foraminifera has been described from Upper Cretaceous to Paleogene deep-water limestones in Italy, Spain (Kuhnt, 1990; Kuhnt & Kaminski, 1989, 1990), and from the Pieniny Klippen Belt in Poland (Bąk, 2000). This assemblage has been termed the "Scaglia-type" biofacies after the type locality, the Scaglia Rossa Formation of the Umbria-Marche Apennines of Central Italy. The oceanic environment represented by the Scaglia Rossa is that of a submarine platform or inter-basinal high, situated away from any detrital influence, with pelagic nannofossil-foraminiferal oozes deposited at lower bathyal to upper abyssal depths, but still above the carbonate lysocline. The sediments at Gubbio consist of rythmically bedded pelagic limestones with occasional chert horizons, and were deposited at a palaeodepth estimated to be between 2,000 and 3,000 meters (Arthur & Premoli-Silva, 1982; Labude, 1984). As the sediments are red in colour, they were deposited under well-oxygenated conditions. No organic carbon values higher than 0.1% occur in the sequence and most values are near 0.01% (Arthur, 1979). The bulk of this sedimentation is made up of primary biogenic carbonate (planktonic foraminifers and nannoplankton). Remains of terrigenous detrital material in grain fractions above 63 microns have not been observed. The clay-mineral content of the Scaglia limestones is low in the Gubbio section. In general, the benthic deep-sea biota seems not to have been affected by terrigenous detrital input or substrate disturbance by turbidity or other bottom currents. Foraminifera from the Scaglia Rossa and Scaglia Variegata can only be studied in acid residues. The agglutinated foraminifera recovered in this manner are surprisingly well-preserved, clean, unbroken, and easy to study. The composition of the Scaglia-type agglutinated foraminiferal assemblage is unique, and comprises not only silicified organically-cemented forms, but also many species that originally possessed calcareous cement and were later subjected to diagenetic silicification. The Scaglia-type assemblages of the Turonian to Maastrichtian of the Scaglia Rossa Formation have been studied by Kuhnt (1990), while the Paleocene/Eocene boundary interval was studied by Galeotti et al. (2004). For the purpose of this study, the entire Scaglia Rossa and Scaglia Variegata section outcropping in the Contessa Road Section near Gubbio has been sampled at 5-metre intervals, and the DWAF picked at >63 microns from 100 g samples.
The DWAF assemblages of the Scaglia Formations contain forms that are normally regarded to be deep water, such as Paratrochamminoides spp. and Cystammina, but curiously the genus Reticulophragmium is very rare. The Scaglia-type biofacies also contains calcareous-cemented forms such as Remesella varians, Karreriella chapapotensis, and Spiroplectammina israelskyi. However, the assemblage also contains an admixture of species that are more typical of the "Flysch-type" assemblages that characterise the more detritic deep-sea environments. These forms include Aschemocella carpathica, Rhizammina spp., Rhabdammina spp., Nothia excelsa, Paratrochamminoides spp., Subreophax spp., Spiroplectammina spectabilis, and Recurvoides spp. These silicified organically-cemented forms are all finely-agglutinated, and the observer gets the impression that the foraminifera were able to gather up all the aeolian-derived detrital silt grains that were present. Perhaps the most distinctive faunal element of the Scaglia-type biofacies is the occurrence of fragile abyssal tubular forms, some of which resemble modern Komokiaceans. Because the depositional environment was obviously tranquil, and because the sample processing technique tends to preserve the fragile forms, the samples from Gubbio contain some unique species. Small tubular forms that lived as encrusting forms or perhaps inhabited the interior of planktonic foraminiferal tests are often observed, as are delicate, branching tubes, and minute specimens of Hyperammina with a long fine tube extending from the proloculus. The deposition of the Scaglia Variegata facies in Italy came to an end during the Late Eocene, when sedimentation became increasingly more detrital.
|
SLOPE MARL BIOFACIES
a. The "mixed" low-latitude calcareous-agglutinated assemblages.
Calcareous claystones and marls deposited above the CCD have been studied from Trinidad, Zumaya Spain, and the Subsilesian Unit of the Polish Carpathians. This biofacies is characterised by varying admixtures of calcareous benthic and planktonic foraminifera, and the DWAF include many calcareous-cemented forms such as ataxophragmiids. The presence of calcareous-cemented species belonging to the genera Clavulinoides, Dorothia, Gaudryina, Marssonella, Popovia, and Arenobulimina contribute to the overall high diversity observed in this biofacies.
Lizard Springs. The cores from the Guayaguarare-287 well in the Lizard Springs Formation studied by Kaminski et al. (1988) contain a mixture of flysch-type faunas in pelagic intervals and redeposited assemblages in the turbidite mudstones. Redoposited assemblages are dominated by Nothia excelsa, with common Clavulinoides, Popovia, Rzehakina, Recurvoides, Ammosphaeroidina, Haplophragmoides (mostly coarse-grained species), Conotrochammina, and Trochammina (listed in order of decreasing abundance). Species of Dorothia, Marssonella, and Arenobulimina are also present. This redeposited assemblage co-occurs with a calcareous benthic assemblage dominated by Stensioeina becariiformis, and was probably derived from a middle bathyal source. This association, dominated mainly by bathyal calcareous-cemented agglutinated species is the locus typicus of our low-latitude slope marl assemblage. Comparision with the paleobathymetry and faunal data of Tjalsma & Lohmann (1983) suggest paleodepths of between 1000 and 2000 metres for this assemblage. The flysch-type assemblages in the pelagic intervals, on the other hand, are dominated by Ammosphaeroidina, Rhizammina, Rzehakina, Recurvoides, Nothia, Spiroplectammina, Hormosina, Saccammina, and Haplophragmoides (mostly fine-grained species).
Zumaya. Our analysis of the Turonian to lower Eocene succession in the Zumaya section (Kuhnt & Kaminski, 1997) yielded over 95 species and taxonomic groups of deep-water agglutinated foraminifera. The whole succession contains foraminiferal assemblages typical of the "low to mid-latitude slope" DWAF biofacies of Kuhnt et al. (1989). Near the base of the lower Campanian, the first occurrences of Spiroplectammina ex gr. dentata and Goesella rugosa are observed. This species is found sporadically in samples throughout the Campanian and Maastrichtian. Species diversity increases in the lower part of the middle Campanian when the first consistent occurrence of Caudammina ovula is observed. Diversity again increases in the lower part of the upper Maastrichtian, when many of the typical "flysch-type" organically cemented agglutinated species appear in the section or become more common. One distinctive event is the FO of Remesella varians in the upper Maastrichtian.
The Cretaceous/Paleogene boundary at Punta Aitzgorri is characterised by an assemblage dominated by organically cemented taxa such as Ammodiscus, Aschemocella, Subreophax, Recurvoides, and the tubular forms Bathysiphon and Rhizammina. Significantly, the LOs of Goesella rugosa and Clavulinoides subparisiensis are both associated with this horizon.
Above the K/P boundary, the DWAF assemblages from the lower Danian carbonate-rich sediments still contain common calcareous-cemented forms such as Arenobulimina, Clavulinoides, and Dorothia. However, higher in the Danian the calciturbidites exposed in the beach outcrop at San Telmo are replaced by more terrigenous sediments, and the organically-cemented flysch-type forms become dominant. The LO of Spiroplectammina ex gr. dentata is observed in Zone P1b. The Paleocene assemblages above this level are dominated by tubular forms such as Rhabdammina, Rhizammina, Nothia, and organically cemented taxa such as Placentammina placenta, Psammosphaera spp., Recurvoides spp, and Paratrochamminoides spp. The DWAF assemblages contain greater proportions of taxa that are typical of greater water depths, and bear a distinct resemblance to the Paleocene Lizard Springs fauna. In his study of the calcareous benthic foraminifera from the upper Palaeocene part of the San Telmo outcrop, Ortiz (1995) also assigned a lower bathyal paleodepth.
The High-latitude Slope biofacies is exemplified by the type of assemblage recovered on the Labrador Margin, for example in the Paleocene of the Indian Harbour and Roberval wells, and in the Eocene of the North Leif and Gudrid wells. Foraminiferal assemblages from these localities were analysed quantitatively by Kaminski (1988).
In contrast to the assemblages from Trinidad and Zumaya, the updip wells on the Labrador Margin are almost devoid of calcareous-cemented agglutinated taxa. In the Paleocene Cartright Formation, Spiroplectinella and Remesella are occasionally found. Instead, the assemblage is dominated by tubular forms with subdominant ammodiscids, lituolids, haplophragmiids, trochamminids, and loftusiids. In the Eocene, tubular forms become rare, and the assemblages are dominated by lituolids, loftusiids, and trochamminids. In the Paleocene, various primitive species of Reticulophragmium become locally abundant, while in the Eocene assemblages may be dominated by Budashevaella multicamerata and Reticulophragmium amplectens. The bathymetrical setting for these assemblages is interpreted as outer neritic to upper bathyal. This biofacies extends from the Northern Grand Banks to the North Sea and into the Arctic (after the middle Eocene).
|
DISTRIBUTION OF THE DWAF BIOFACIESIn the broadest general terms, the distribution of the four main DWAF biofacies can be related to simple bathymetric terms, in as much as they characterise different physiographical provinces in the ocean (Fig. 5). The deepest biofacies, the abyssal "Celebes fauna" of the abyssal plains is restricted to DSDP/ODP sites with backtracked palaeodepths exceeding 4 km. However, more importantly, this fauna occurs at sites that were located away from the continents, for example on ridge flanks and abyssal plains. The Scaglia-type biofacies was also isolated from terrigenous influence, but was deposited on submarine highs and oceanic platform deposits. These assemblages characterise the pelagic environments between 2 and 3 km depth. In contrast, the flysch-type assemblages from the base of the continental rise in the Atlantic, in the Carpathian flysch troughs and in the North Atlantic petroleum basins are comprised of different species, and characterise clastic-dominated substrates. The continental rises and small tectonically active basins are the areas where the flysch-type faunas are best developed. This biofacies as such covers a wide bathymetric range of clastic environments beneath the CCD. Above the CCD, the areas that receive marly deposition are characterised by the Slope biofacies. Areas such as seamounts, or the bathyal zones of the continental margins receive calcareous sediments, and in addition to the flysch-type taxa, the calcareous-agglutinated biofacies is well developed in these areas. Towards the north, the CCD shallows and the typical low-latitude Slope biofacies gives way to the Boreal Slope biofacies in which calcareous-cemented forms are absent or rare. However, it must be borne in mind that during the Paleocene, the CCD was much shallower in the Atlantic than it is today. For a number of reasons, the water depth represented by these assemblages is not constant, and the bathymetric distribution of a given the biofacies varies owing to changes in the environment (e.g., depth of the CCD, palaeoproductivity, oxygenation, nature of the substrate). The general features of the main agglutinated foraminiferal biofacies are summarised in figure 5.
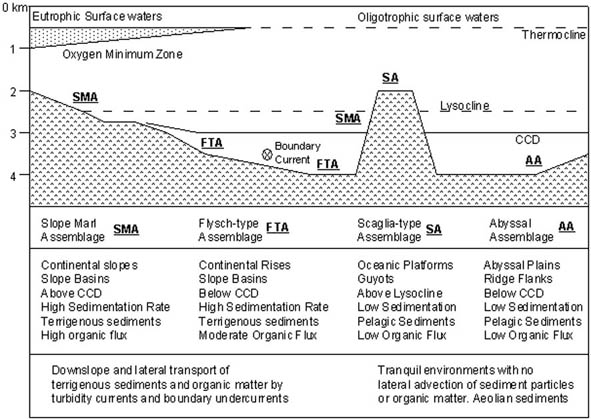
Figure 5. Environmental summary of deep-water agglutinated foraminiferal biofacies in the Paleogene ocean. Back on top | Abyssal | "Flysch"-Type | Scaglia-Type | Slope Marl | Relation to Physical Parameters |
RELATION WITH PHYSICAL ENVIRONMENTAL PARAMETERS
a. Relation to Substrate
All four DWAF biofacies display a response to substrate parameters at least to some degree. The presence or absence of particular species may be controlled by the availability of particular sedimentary particles. In the modern ocean, for example, Williamson (1983) was able to quantify the distribution of modern benthic foraminifera in the North Atlantic, and demonstrate that some calcareous benthic species displayed correlations to sediment grain size parameters.
Abyssal assemblages that live on and within deep-sea pelagic clays are comprised of finely agglutinated forms. Coarsely agglutinated forms are notably absent. It has been observed, however, that some modern finely agglutinated species will use coarse grains if these are made available. Deep-sea forms normally agglutinate the fine-grained aeolian quartz silt that finds its way to the deep ocean, but the case of the Mt. Pinatubo volcanic eruption has demonstrated that some species clearly have preferences for other mineral phases as well (e.g., Hess et al. 2000).
Scaglia-type assemblages also display a type of substrate dependence. In acid residues, we observe a variety of tubular forms that lived attached to empty planktonic foraminiferal tests, and even within these tests. Attachment surfaces are observed on various tolypamminids. In the modern ocean, several authors have reported DWAF that live attached to empty foraminiferal tests or internally, within tests. Other species, such as Psammosphaera testacea, utilise juvenile planktonic foraminiferal tests as agglutinated particles, while a number of tubular forms pick up sponge spicules. Slope marl assemblages contain a number of species that use calcareous particles in the construction of their tests, while this feature is not noted in the abyssal assemblages. The presence of suitable pre-existing biogenic particles is a prerequisite in this case.
Flysch-type and slope marl assemblages require terrigenous clastic material to construct their tests. Coarsely agglutinated forms such as Ammobaculites, Psammosphaera fusca, and the large-diameter tubular forms only occur along the continental margins and in the flysch basins. In the southern Atlantic and southern Ocean, Psammosphaera-dominated faunas have been found on winnowed substrates beneath the axis of the strong current of Antarctic Bottom Water that flows through the Scotia Sea and northward along the Argentine continental rise (Harloff & Mackensen, 1997). A Paleocene example of a Psammosphaera-dominated assemblage was observed in the basal part of the Torsk Formation in the western Barents sea wells, in coarse sediments just above a hiatus (Nagy et al., 1997).
The standard explanation that DWAF assemblages represent "a deep environment below the CCD" is misleading, because the CCD is by no means a static or constant horizon. In the modern ocean the depth of the CCD varies from 5.5 km in the western North Atlantic to as shallow as 2.5 km in the North Pacific (Berger & Winterer, 1974). During the early Paleogene the CCD probably extended up to shelf depths in the high latitude basins such as the Barents Sea and Arctic. Its depth is more closely linked to the flow pattern of deep ocean circulation (the deep ocean conveyor), the availability and flux of biogenic calcium carbonate, the residence time of deep water in the world ocean, and the rate of respiration below the photic zone, which is in turn related to surface productivity.
During the Late Cretaceous to Paleocene the CCD was high for a variety of reasons. Sea level was high and carbonate production occurred over the shelves rather than in the deep ocean. Moreover, ocean circulation was sluggish, and bottom water was undersaturated with respect to calcium carbonate. A rise in the CCD has the effect of opening up more habitat space for the abyssal agglutinated faunas to colonise vast areas of the sea floor. Conversely, at times when the CCD fell, DWAF diversity is observed to decline.
A widespread rise in the CCD took place at the Paleocene/Eocene boundary, and once again during the latest early Eocene. This is best expressed in the Contessa section of northern Italy (Galeotti et al., 2004) at ODP Site 647 in the Labrador Sea (Kaminski, 1988). During both events, a "Glomospira assemblage" is found denoting a period of fauna turnover. The P/E boundary sees the extinction of several species, notably Caudammina ovula and some primitive Reticulophragmium, while the early Eocene event is marked by the last occurrence of Caudammina ovuloides and the first occurrence of others. The CCD fell significantly at the Eocene/Oligocene boundary, reflecting better ventilation of the deep ocean associated with climatic cooling and the onset of Antarctic glaciation. At this time, agglutinated foraminiferal faunas were largely replaced by calcareous ones. For example, at Site 647, where the boundary interval was continuously cored, some 12 species of DWAF disappeared from the record, including S. spectabilis. It is possible that in carbonate-rich environments the calcareous benthics might outcompete the agglutinates, but to what extent the reduction of habitat space beneath the CCD causes extinction of DWAF species still remains unclear.
The idea of a close relationship between the shape of the foraminiferal test and the preferred microhabitat follows from early speculation on test function by Edward Heron-Allen (1915), who should be rightly named the "Father of Foraminiferal Functional Morphology". Heron-Allen realised that different test shapes and the selection of particular agglutinated particles are adaptations to life in particular microenvironments He speculated that for example, the single long sponge spicule used by Psammosphaera parva may "serve as catamaran spars in supporting the animal on the surface layer of the ooze". Likewise, he believed that the sponge spicules found near the apertural openings of Marsipella and Haliphysema "must prove a very efficient protection against parasitic worms". For lack of a better word to describe these examples of grain selectivity and functional morphology, Heron-Allen termed this "the phenomena of purpose and intelligence".
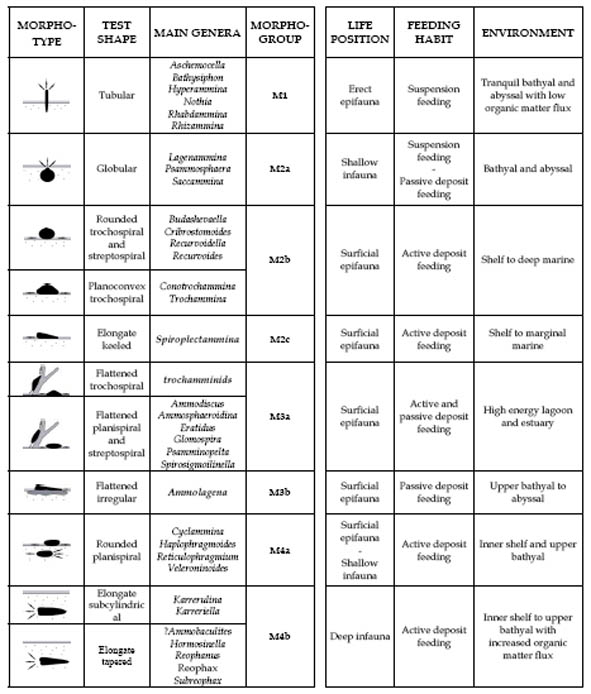
The modern concepts behind the idea of functional morphology in agglutinated foraminifera stems from the synthesis paper of Jones & Charnock (1985). These authors devised four morphological groups (termed "morphogroups") that related test shape to preferred trophic habitats. The "morphogroup concept" has been refined by subsequent authors (e.g., Nagy et al., 1995),
and an additional trophic group, the "phytodetritus opportunists" has been described subsequently (Gooday, 1993). It is possible that other types of feeding strategy (such as parasites or predators) also exist as in other groups of protozoans. Importantly, some species such the Antarctic tubular forms may utilise more than one feeding strategy. It has even been noted that other tubular forms such as the Komokiaceans may harbour endocytoplasmic bacteria. Nevertheless, the DWAF can be broadly grouped into four main groupings based upon gross morphology (Fig. 6). Some of these morphogroups can be further subdivided into morphotypes.
Figure 6. Definition of agglutinated foraminiferal morphogroups, with interpreted life habitat and feeding strategy (modified from Nagy et al. 1995, and Van den Akker et al., 2000)
Morphogroup 1. This morphogroup consists of suspension-feeding epifaunal forms that live attached to the substrate and extend their pseudopodial networks into the water column to collect food particles. One example is the genus Saccorhiza, which has been found to deploy its pseudopodia into the benthic boundary layer, at the boundary between lamellar and turbulent flow where suspended particles tend to accumulate (Altenbach et al., 1988). We have observed Psammosiphonella in life position in multicores collected from the South China Sea (Kaminski & Wetzel, 2004a). Suspension-feeding organisms are often found in areas that have gentle currents. Forms belonging to Morphogroup 1 are most common in lower bathyal to abyssal environments (Schröder, 1986), where the deep boundary currents bring a constant supply of suspended food particles. They can also take advantage of any opportunity to capture suspended particles, such as growing near the entrance of a shrimp burrow (Linke & Lutze, 1993)
Morphogroup 2 comprises the epifaunal detritivores that live on the sediment surface or just below it. This group most likely feeds on bacteria and detritus from the flocculent layer (Nagy, 1992). The group can be further subdivided into morphotypes based on differences in gross morphology. The epifaunal detritivores are trochospirally coiled motile forms or spherical forms that may extend their pseudopodia to the sediment surface. Modern spherical forms such as Astrammina rara appear to be stationary and live just beneath the sediment surface, but extend their pseudopodia up to the surface to feed (De Laca, 1988). Keeled forms with mixed coiling modes (Spiroplectammina and Spiroplectinella) are also listed here. Some tubular forms, however, are classified as epifaunal detritivores. The branching forms such as Aschemocella, Rhizammina, or Nothia excelsa form networks on the sediment surface. In some cases just the tips of the tubular astrorhizids may be upturned and extend above the sediment surface.
Morphogroup 3 describes the flattened morphotypes. The first morphotype (3a) consists of trochospiral epiphytal forms such as the shallow-water trochamminids that live attached to marsh plants. These forms were considered by Jones & Charnock to be herbivores, and are most common in shallow-water assemblages. In the deep sea, attached trochamminids do exist - one of the most spectacular of which is the genus Abyssotherma, which lives in the proximity of deep-sea hydrothermal vents (Brönnimann et al., 1989). Deep-water representatives of this morphotype include flattened planispiral and streptospiral forms such as Ammodiscus, Glomospira, and Rzehakina. Modern Ammodiscus has been found to be an active epifaunal scavenger (Bornmalm et al., 1997). The second morphotype (3b) comprises attached epifaunal forms such as Ammolagena.
Morphogroup 4 consists of deep burrowing infaunal forms, and includes two morphotypes. The planispiral forms with a rounded periphery (such as Haplophragmoides and Reticulophragmium) are placed in morphotype 4a. Calcareous genera possessing this shape (e.g., Melonis) occup the shallow infaunal habitat (Corliss, 1985). The majority of infaunal forms (morphotype 4b) are uniserial or multiserial and have tapered, elongated, streamlined shape, such as found in the genera Reophax, Karrerulina, and Remesella. These forms have been found at depths of up to 15 cm below the sediment surface, and can certainly live beneath the Redox boundary. Typically, they dominate in low-oxygen environments, and can even tolerate periods of total anoxia. Some infaunal forms have been found to be deposit feeders that live by cropping the bacteria that occur in abundance just below the Redox boundary. These forms are generally adapted to life in more eutrophic areas, with higher levels of organic flux. Another possible food resource for the infaunal species might be food caches within worm burrows, or bacteria associated with burrows.
Another rather small group of species that do not really fit into the morphogroup/feeding strategy model consists of "phytodetritus opportunists", such as the modern species Adercotryma glomerata. These forms apparently reproduce rapidly, and are found living within the seasonal fluffy phytodetritus layer that appears on the floor of the North Atlantic during summer (Gooday, 1988, 1993). No doubt other feeding strategies also exist among deep-sea agglutinated foraminifera, such as direct uptake of dissolved organic matter. Finally, forms such as the Antarctic genus Nothodendroides apparently utilise more than one feeding strategy, feeding as a suspension feeder during the summer phytoplankton bloom, but as a detritivore during the "leaner" times of the year.
The use of morphogroup analysis for palaeoenvironmental interpretation of agglutinated assemblages has been the subject of many papers since the beginning of the 1990s. The basic concept behind the analysis can be compared to the once-infamous "bread-queues" in Eastern Europe during the waning years of the Soviet administration. Basically, if you can extend your pseudopodia into the water column and capture falling food particles, you are at the front of the queue and you get your bread. Whatever escapes the "spiderweb" of the suspension feeders is snapped up by the epifaunal detritivores. Any organic matter that is left over becomes incorporated into the sediment (aided by macrofaunal activity) and eventually serves as food for the infauna, either directly or by sustaining bacterial populations. It is a general pattern that the modern deep ocean (below about 1,500 m) is populated mostly by epifauna, while the more eutrophic or productive regions harbour high percentages of infauna (see Corliss & Chen, 1988; Corliss & Fois, 1991). Competition between the epifaunal and infaunal morphogroups may also be an important unknown factor, and the transition between epifaunal-dominated and infaunal-dominated communities is often abrupt rather than transitional. Morphogroup analysis is then a quick and easy method for identifying changes in organic flux to the sea floor, and as such can serve as a palaeoproductivity proxy.
Attempts at applying morphogroup analysis to DWAF assemblages have generally yielded results in line with predictions, for example Kuhnt & Kaminski (1993, 1996) found an increase in epifaunal forms above the K/P boundary; and Nagy et al., (1997) documented a maximum in tubular forms (Morphogroup 1) across the Paleocene-Eocene transition in the Western Barents Sea, reflecting reduction in organic flux. In the Miocene, Preece et al., (1999) found a relationship between the infaunal morphogroup and total organic carbon values in an offshore exploration well off Cabinda, West Africa. As our knowledge of the benthic foraminiferal niche incrementally improves, there is little doubt that the morphogroup analysis can be further refined.
Substrate disturbance plays an important role in the ecological structuring of benthic communities. According to some theoretical models, episodic disturbance is necessary to maintain high taxonomic diversity in benthic communities. Disturbance may be of two kinds: physical, caused by contour currents, turbidity currents, benthic storms, volcanic ashfalls, or anoxia; and biological, caused mainly by predation or the burrowing activities of larger animals. Most studies on deep-sea benthic foraminifera have focussed on the role of physical disturbance.
Disturbance is defined as any environmental factor that causes mortality, whether it is selective or in mass. Winnowing by bottom currents is an example of selective mortality, as in the case of the High Energy Benthic Boundary Layer Experiment site, at 4815 m off Nova Scotia. At this locality, the interaction between a deep western boundary current and meanders and rings of the Gulf Stream cause locally strong bottom currents that can resuspend sea floor sediment to a depth of several centimetres. In this area, populations dominated by infaunal species of Reophax and Psammosphaera were found living in newly deposited surficial sediment (Kaminski, 1985). These were interpreted as opportunistic forms, as they can repopulate newly deposited sediment. Either some species of DWAF can survive being resuspended, or the surviving infaunal community moved upwards to colonise the new substrate. Additionally, resuspension and transport of opportunistic forms by bottom currents is a mechanism for dispersal. Living specimens of Reophax scotti have been found in plankton tows collected from the North Sea (John, 1987). These organisms may have gone into suspension in shallow water areas during a major storm.
A similar type of agglutinated foraminiferal community dominated by Reophax and Psammosphaera was observed in recolonisation trays placed in the deep Panama Basin (Kaminski et al., 1988). After nine months on the sea floor, sediment trays contained living specimens of R. dentaliniformis, R. excentricus, and Psammosphaera fusca, which must have colonised trays from suspension or by crawling into them. No living tubular forms were found, suggesting that immobile forms have a more limited capability of disperal.
In a study of the deep San Pedro Basin off southern California, an area that experiences seasonal anoxia, Kaminski et al. (1995) reported a remarkably similar faunal assemblage consisting of Psammosphaera, Reophax dentaliniformis, Reophax spp. and a minute organically-cemented species of Textularia that was interpreted as opportunistic. Many of the species were the same as those found in recolonisation trays in the Panama Basin, suggesting that different types of disturbance may result in similar benthic foraminiferal communities.
The recovery of DWAF after mass mortality caused by volcanic eruptions has been documented by Hess & Kuhnt (1996), Hess et al., (2001) and by Galeotti et al., (2002). In what is a natural laboratory to study the process of recolonisation, the South China Sea, Hess & Kuhnt have documented the first pioneering species that are currently colonising the surface of the 1991 Mt. Pinatubo tephra layer. The eruption of Mt. Pinatubo volcano deposited up to 8 cm of volcanic ash on the sea floor adjacent to the volcano. At stations where the ash thickness exceeds 2 cm, reduced diversity of the surficial fauna (compared with the pre-ash assemblages) is observed. When the ash layer was first sampled in 1994, most of the specimens on top of the ash were alive, meaning that Hess & Kuhnt (1996) had sampled the first generation of the recovery fauna. The first recolonisers were species regarded as mobile, infaunal detritivores, some of which have been previously reported from physically disturbed deep sea environments. Typical representatives of this group include Reophax bilocularis, Reophax dentaliniformis, Textularia earlandi, and the calcareous species Bolivina difformis and Quinqueloculina seminula. Lacking predation and competition in the newly occupied habitat, they rapidly reached high standing stocks, indicating rapid reproduction. A second succession of colonisers appeared between 1994 and 1998. Hormosinelloides guttifer, Trochammina spp. and Adercotryma glomerata were the dominating species in the living fauna in 1996-1998, while the dead assemblages were dominated by the pioneer colonisers (Hess et al., 2001). The diversity of the fauna significantly increased during this time. The first epifaunal tubular foraminifera (including Rhizammina, and large specimens of Astrorhiza crassatina) appeared, and xenophyophorians started to flourish on the sediment surface. Seven to eight years after the ashfall, the trochamminid group (Trochammina ex gr. globigeriniformis, Trochammina sp., Adercotryma glomerata) has increased markedly in abundance, and coiled multichambered forms such as Recurvoides, Eratidus, Ammobaculites, Haplophragmoides, and Karrerulina are beginning to appear in the samples. Diversity values of the recolonising fauna are still lowest near Mt. Pinatubo, and increase in a distal direction away from the volcano, which implies that recolonisation takes place first along the outer edges of the tephra layer.
The process of faunal decimation and recovery following tephra falls must undoubtedly be a common occurrence in the geological record, contributing to local differences in the patch structure and successional patterns observed in deep-sea benthic foraminifera. However, a major unknown question is whether or not this process leaves an observable fossil record, and if so what is the timescale of faunal recovery in the deep sea? Galeotti et al. (2002) addressed these questions by documenting the pattern of the recolonisation through a high resolution study of DWAF from below, within, and above a 15 cm-thick volcanoclastic layer in the Scaglia Rossa Formation exposed in the Furlo Gorge (Umbria-Marche Apennines, Central Italy). Below the volcanoclastic layer, DWAF assemblages are diverse with 28 to 34 species, and only minor fluctuations in the relative abundance of taxonomic groups and faunal parameters are observed. Common taxa are Paratrochamminoides spp., Trochamminoides dubius, Trochammina spp., Subreophax spp., Saccammina spp. and tubular forms.
The recolonising assemblage above the bentonitic layer is significantly different, showing reduced diversity and an increased proportion of infaunal forms. High relative abundances of Scherochorella minuta, Pseudobolivina cf. munda, (the latter being almost exclusively represented just above the volcanoclastic layer) are observed in the first centimetre above the bentonitic layer, in association with juvenile/dwarfed specimens of Paratrochamminoides and Trochamminoides. Between two and four centimetres above the volcanoclastic layer, the highest relative abundances of Subreophax scalaris, S. splendidus, Hormosinella cf. distans, and Karrerulina sp. are observed. These taxa represent the next stage in the recolonisation process, and although exploiting niches opened up by the mass mortality of the original fauna, are not as opportunistic as S. minuta and P. cf. munda. The recolonisation fauna is comprised mainly of infaunal morphologies, which apparently live at the sediment surface in disturbed conditions. The succession of species above the volcanoclastic layer at Furlo displays interesting similarities to the succession of species found colonising the 1991 Mt. Pinatubo tephra layer in the abyssal South China Sea. Profound differences between the calculated duration of the recolonisation process at Furlo and that observed in the South China Sea are attributed to bioturbational mixing within the Scaglia Rossa, which resulted in an expanded record of the fossil "recolonisation assemblage". Despite the passage of 80 million years of Earth History, broad similarities at the generic level between modern and fossil analogues strengthens the reliability of environmental reconstructions based on DWAF.
The observation that benthic macrofaunal invertebrates prey upon benthic foraminifera is long established (Lipps & Valentine, 1970; Sliter, 1971). In the deep sea, benthic foraminifers and other protozoans make up a significant proportion of the biomass, and serve as prey items for numerous macrofaunal organisms (Sokolova, 1997). Already in the last century the gut contents of holothurians collected during the HMS Challenger Expedition were found to contain large numbers of benthic foraminifera. More recently, Svavarsson et al. (1993) found benthic foraminifera in the gut contents of two species of isopod crustaceans (Ilyarachna hirticeps and Eurycope inermis) in the Norwegian Sea, Langer et al. (1995) found selective predation of foraminifera by the deep-sea scaphopod Fissidentalium megathyris off California, and Sokolova et al. (1995) found agglutinated foraminifera in the gut contents of four species of abyssal ophiurids collected from the Orkney Trench of the Southern Ocean. According to Sokolova (1997), agglutinated protozoans belonging to the group of xenophyophoreans and komokiacean foraminifera may constitute up to 40% of the gut contents in some abyssal macrobenthos.
During one of the South China Sea cruises, we encountered an open burrow in one of the box cores that was filled with specimens of agglutinated foraminifera (Kaminski & Wetzel, 2004b). The burrow was onion-shaped, with an inclined entrance tube (0.5 cm diameter) that was open to the sea floor. The burrow was constructed in abyssal brown clay beneath the 1991 Mt. Pinatubo ash layer The burrow was similar in overall morphology and dimensions to the burrows and feeding cavities created by maldanid polychaetes ("bamboo worms") found by Levin et al. (1997) on the Carolina slope. However, the lack of an agglutinated burrow lining suggests that a different animal constructed the burrow, possibly a crustacean (Lisa Levin, personal communication to MAK, 1999). In the sectioned core, the agglutinated protozoan-filled part of the burrow was observed about 8 cm beneath the sediment surface, and the chamber was 2.5 to 3 cm in diameter. A total of 83 specimens of Aschemonella ramuliformis and Psammosiphonella discreta filled the chamber of the burrow completely. Only a few specimens of non-tubular agglutinated foraminifera were found, including one specimen each of Ammodiscus sp. and Cyclammina cancellata. The tubular tests appeared to be undamaged, up to 2.5 cm long, and in random orientation. To our knowledge, this is the first report of such evidence of selective predation on tubular agglutinated foraminifera. We assume the burrow occupant made regular forays to the sea floor to search for prey items, bringing specimens back to its burrow, a behaviour known as "caching" (Jumars et al., 1990). There is now no doubt that deep-sea agglutinated foraminifera constitute a key link in the metazoan food chain, and that the patchiness in their distribution reported by many workers may be attributed to biological causes.
Palaeobathymetry of microfossil assemblages is an important parameter in the tectonic subsidence analysis and deposition history of well sites, and is widely applied in petroleum basins to strengthen models of reservoir and source rock sedimentation and distribution. Estimates of changes in palaeobathymetry also detail the amount of sediment underfill in basins, and assist with palaeogeographic reconstructions. Sudden changes in palaeobathymetry due to accelerated subsidence or uplift may explain why assemblages disappear (like many agglutinated taxa during basin shallowing), and often modify the stratigraphic ranges of many taxa.
An overestimation of palaeo waterdepth may induce artificial uplifts through time in well site subsidence models, whereas its underestimation artificially reduces subsidence rates. Ignorance of palaeobathymetric trends will generally lead to an underestimation of basin subsidence, and incorrectly assigned palaeoslopes.
Ignorance of palaeobathymetric trends in basin analysis is not uncommon in the geological literature. Reasons may be many, but not in the least because palaeobathymetry is a bit of a fearful subject. The science, or should we say 'the art of the science', tries to extract rigorous geometric information, i.e. the height of the palaeo watercolumn at discrete time intervals, from organisms that do not per se sense depth. The presently most widely employed and probably most detailed palaeobathymetric techniques are of a micropalaeontological nature, particularly involving benthic foraminifera in Jurassic, Cretaceous and Cenozoic strata. Although there is a widespread consensus among micropalaeontologists that the benthic taxa are roughly arranged along a depth-, and down to basin gradient, there are no easy rules and several constraints that hamper accurate predictions (van der Zwaan et al., 1999), including:
It is generally agreed that food availability (including bacteria), the redox gradient, competition for food and space, and a host of physical, chemical and environmental setting factors together are responsible for determining the upper depth limits and assemblage composition of agglutinated assemblages. But a thorough theoretical and quantitative basis for palaeoecology of foraminifera is still in its early stage. It is clear that water depth and export production (including seasonality of export production) both determining the amount of organic carbon arriving at the sea floor. Recent studies have quantified the relationship between sea floor carbon flux and modern foraminiferal facies in the eastern Atlantic and Mediterranean (Altenbach et al. 1999; De Rijk et al., 2000). A general pattern is appearing which links both upper depth limits of individual species and assemblage composition to food availability. However, sea floor oxygenation and disturbance by bottom currents can also affect assemblage composition. That is where empirical models take over that create practical rules of thumb in direct interplay with the palaeoceanographic, geologic and palaeogeographic setting of geological basins. With respect to agglutinated foraminifera three observations stand out:
We will return to the dissolution scenario, following a discussion of an empirical approach to palaeobathymetry, involving agglutinated foraminiferal assemblages in two petroleum basins, i.e., the Cenozoic sedimentary wedges off northeast Canada, and in the North Sea.
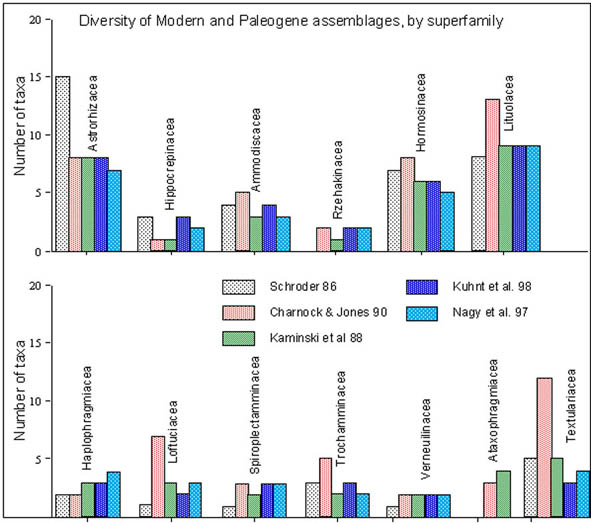
Paleogene sediments of the Central North Sea and the Canadian Atlantic margin abound in predominantly agglutinated benthic foraminiferal assemblages. The fauna as a whole, probably is made up of over 150 species and 75 or more genera from all major (approximately 9) superfamilies (Fig. 7), a majority using organic cement to create a test from sand grains. Over the years, problems have arisen with interpretation of palaeo waterdepth based on the assemblages that make up this fauna, largely from insufficient insight into the modern niche the taxa prefer, and lack of detailed observations along basinward transects in siliciclastic basins.
Figure 7. Number of genera of agglutinated foraminifera organised per family in the Recent North Atlantic slope and abyssal plains (Schröder, 1986), in the Paleogene of the North Sea (Charnock & Jones, 1990), the Paleocene of Trinidad (Kaminski et al. 1988), the Paleocene of West Africa (Kuhnt et al. 1998) and the Paleocene of the western Barents Sea (Nagy et al. 1997).
As pointed out in a number of studies (Gradstein & Berggren, 1981; Miller et al., 1982; Scott et al., 1983;
Schröder, 1986), the diverse and abundant agglutinated species found in wells that sampled the Paleogene sediments of the North Sea, offshore Norway, and offshore Eastern Canada, at present occur mostly on the modern continental slope, with their niches ranging down onto the abyssal plain.
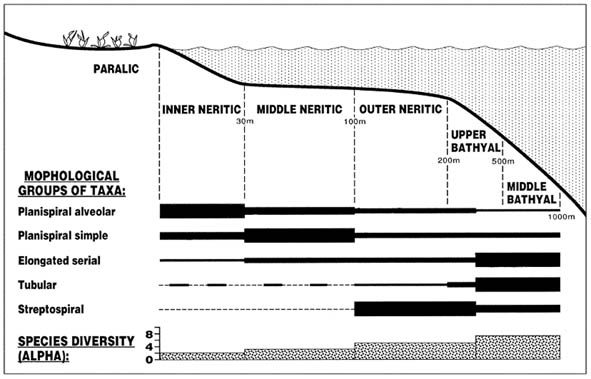
The modern fauna, which generally includes more common calcareous than agglutinated benthic taxa, favours a fine-grained siliciclastic, low energy substratum, which is why its fossil counterparts predominate in mudstone basins where sediments may also be generally enriched in organic matter, and petroleum prone. The modern agglutinated fauna is not strictly bathymetrically controlled. On the other hand, the so-called flysch-type fauna, which was named after assemblages that are typical of some of the classical "flysch"" deposits in the Carpathians and Alps (Gradstein & Berggren, 1981), from detailed comparisons with the modern slope fauna (Scott et al., 1983; Schafer et al., 1983; Schröder, 1986) has an upper depth limit of around 500 m. Some of the individual genera may be found on the shelf, or even in marshes (e.g., some species of Trochammina or Ammobaculites), although on a species level there is limited overlap between modern shelf and slope agglutinated faunas (Fig. 8).
Figure 8. Distribution of selected species of agglutinated foraminifera in the Recent northwest Atlantic marshes, estuaries, shelf, slope and abyssal plain. The total number of agglutinated taxa increases dramatically from marsh to slope (modified after Scott et al., 1983, and Schröder, 1986).
In our concept of "The Recent as a Key to the Past", augmented with observations along palaeoslope transects (see below), the agglutinated benthic assemblages in Paleogene strata of North Sea and Canadian offshore wells are typical for middle to upper slope conditions. From Recent observations, taxa such as Cystammina, Buzasina, and Pseudobolivina are more typical of middle to lower slope conditions, which agrees with their absence in more updip, basin margin wells studied.
In order to strengthen palaeobathymetric interpretations, Gradstein et al. (1994) analysed faunal trends along palaeoslope transects, using well data and long seismic lines in the subsurface of both the Central North Sea, and northern Grand Banks. For the Canadian Atlantic margin, we also took into account seismic and well transect data on the Labrador Shelf. The objective was to assess lateral faunal changes along shelf to basin transects, and create palaeobathymetric models for these petroleum basins that could be used in burial and subsidence analysis. Palaeoslope studies estimated the deepest part of the transects to be between 750 and 1000 m water depth.
Spatial and temporal trends observed in the agglutinated benthic fauna along the transects may be summarised as follows: Well and seismic data indicate rapid mid Paleocene deepening of the North Sea basin, following deposition of the Danian chalk. As a result of this deepening, that resulted in fine grained mudstones and gravity flow sand wedges, a flysch-type fauna invaded the basin. After the Paleocene, progressive shallowing and delta top facies along the basin margins did not provide a suitable environment for the agglutinated fauna. More centrally in the basin, the agglutinated benthic fauna (with associated radiolarian blooms), becomes much more diversified, and also extends into younger strata. The latter reflects the fact that sufficiently deep marine (bathyal) conditions with fine grained substrate were maintained towards the centre of the basin into Miocene time. During the Miocene rapid shallowing in the central North Sea led to displacement of the agglutinated associations with a neritic, calcareous benthic fauna.
Among the agglutinated benthic taxa, cyclamminids, rzehakinids, cystamminids, Recurvoides and Karrerulina are confined to the basinal wells, with Bathysiphon, Haplophragmoides, Cribrostomoides, Ammodiscus, Glomospira and Saccammina being more opportunistic and extending to the margin. Pseudobolivina, Rzehakina, and (abundant) radiolarians, like "Cenosphaera", are also confined to the basinal mudstones. In general, the number of taxa of the family Lituolidae increases at the expense of the Ammodiscidae. Comparable trends were observed in the Cenozoic siliciclastic wedges in the eastern Canadian offshore.
The above observations are in agreement with those of Jones (1988), who analysed agglutinated faunal changes along a Viking Graben palaeodepth transect, parallel to ours, but slightly to the north in the Frigg petroleum field area. Jones (1988) estimated the so-called basin floor assemblage to be 1000-1500 m deep, with our estimate favouring the shallow end of this bracket. There is likely to be an uncertainty in the palaeo waterdepth estimate that may exceed 25% of the depth estimate on the middle to lower slope.
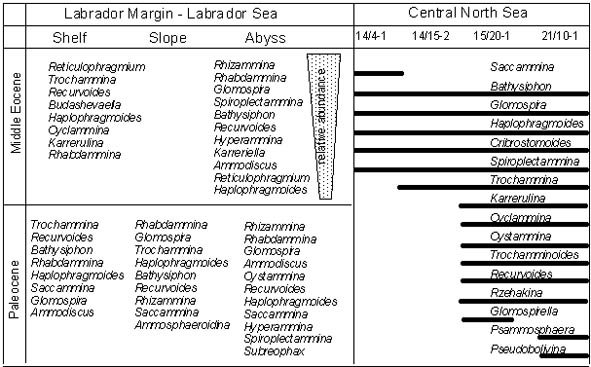
In order to summarise the agglutinated faunal trends along the Grand Banks and the North Sea transects from a shallow, proximal to deeper, distal facies, we have listed observed taxonomic and diversity trends in figure 9. Clearly, both abundance and diversity in taxa dramatically increase down slope. There are fewer than 10 genera and 15 species up slope and routinely 15-30 genera and 30-60 species or more down slope. Bathysiphon, Glomospira, Saccammina, Ammodiscus, Haplophragmoides, Cribrostomoides and Trochammina are more opportunistic and widespread. On the Labrador Margin Trochammina, Recurvoides, Reticulophragmium and Budashevaella are more abundant at shelf localities, while Rhabdammina, Cyclammina, Kalamopsis, Lituotuba, Hormosina, Ammosphaeroidina, Cystammina, Recurvoides, Karrerulina, Rzehakina, Pseudobolivina and Labrospira together are more typical for middle or lower slope conditions. At DSDP Site 112 in the Labrador Sea, Paleocene abyssal assemblages contain finely agglutinated tubes, Glomospira irregularis, small ammodiscids, Cystammina, Spiroplectammina and Subreophax. In the Eocene, faunal diversity is noticeably higher at ODP Site 647 than in exploration wells on the Labrador margin. Although many species are isobathyal, there are important differences in relative abundance (Fig. 9). The abyssal assemblage consists largely of tubular forms, with high proportions of Glomospira and Spiroplectammina. The latter forms have a diachronous occurrence in the Labrador Sea, disappearing in the Lower Eocene along the margin, but extending to the Oligocene at the abyssal location. Abyssal taxa found only at Site 647 include the species Ammodiscus nagyi, Duquepsammina cubensis, and minute specimens of Hyperammina. As indicated earlier, there appears to be a local water depth difference of 500-1000 m basinward in the North Sea, which provides a (minimum) estimate of water depth difference between faunal end members.
Figure 9. Bathymetric distribution of selected, coarse agglutinated benthic foraminiferal taxa in Paleogene fine grained, deep-water sediments, North Sea/offshore Norway and Canadian Atlantic margin (after Kaminski, 1988; Gradstein et al., 1994). Taxa are ranked in order of decreasing relative abundance.
The agglutinated Foraminifera from neritic to bathyal facies in the Paleogene of Spitsbergen and the Barents Sea were studied by Nagy et al. (2000). High latitude Paleogene foraminiferal assemblages were analysed from two onshore sections, located in the Central Basin of Spitsbergen, and from an exploratory well drilled in the Tromsø Basin of the southwestern Barents Sea. A quantitative comparison of the assemblages revealed consistent faunal trends that can be related to palaeobathymetry. The inner and middle neritic assemblages of the Central Basin are of a restricted marine nature as typified by low diversities and virtually entirely agglutinated assemblages. The outer neritic to middle bathyal assemblages of the Tromsø Basin are of a "flysch type" cosmopolitan nature, and are unaffected by high latitude endemism typical of shallower assemblages. These deeper water assemblages reveal close similarities to Paleogene faunas of the North Sea and Tethyan localities. The following foraminiferal biofacies were recognised:
An inner neritic biofacies is found in the Central Basin of Spitsbergen, where it is developed in the Kolthoffberget Member of the Firkanten Formation. The member consists of interbedded shales, siltstones and sandstones deposited in a transitional delta front - prodelta environment. The foraminiferal assemblages are entirely agglutinated, show extremely low species diversities and strong dominance of Reticulophragmium arcticum followed by Labrospira aff. turbida.
A middle neritic biofacies is found in the Basilika Formation (Central Basin) which was deposited under delta-influenced shelf conditions. The foraminiferal assemblages show an increased species diversity, and consist mainly of agglutinated taxa with a strongly subordinate calcareous component. The two most abundant species are R. arcticum and L. aff. turbida. Species of Verneuilinoides and Trochammina occur in significant numbers while tubular taxa are rare.
An outer neritic to upper bathyal biofacies is present in the upper Torsk Formation of the Tromsø Basin. It contains entirely agglutinated assemblages with comparatively high diversities. The dominant species are Recurvoides aff. turbinatus and Budashevaella multicamerata, while Reticulophragmium amplectens is typical and common in these strata. Tubular forms referred to Rhizammina occur locally in significant quantities. The diversity and composition of the assemblages suggest an outer neritic to upper bathyal environment.
An upper-middle bathyal biofacies is recognised in the lower part of the Torsk Formation (Tromsø Basin). The assemblages reveal closest similarities to deep water "flysch type" faunas of the North Sea and other boreal areas. Dominant species include: Spiroplectammina spectabilis, Haplophragmoides walteri, Recurvoides sp. 1, Ammosphaeroidina pseudopauciloculata and Saccammina grzybowskii.
The distribution trends of general faunal parameters and major morphological groupings (Fig. 10) appear to be useful for distinguishing the various foraminiferal biofacies. A number of general faunal trends are apparent in the data from Spitsbergen and the Barents Sea:
Figure 10. A summary of the palaeobathymetric distribution of foraminiferal groups and species diversity for the Paleogene of the Central Basin of Spitsbergen and the Tromsø Basin in the southwestern Barents Sea (from Nagy et al., 2000).
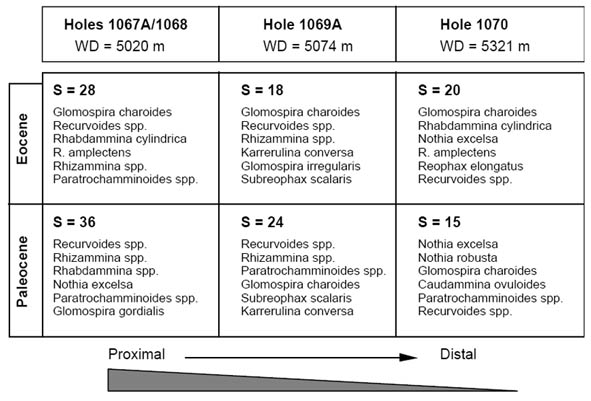
The palaeobathymetrical model for the truly abyssal Paleogene assemblages in the North Atlantic is based on the detailed work of Kuhnt & Urquhart (2001) from the depth transect drilled during ODP Leg 173 on the southern Iberia abyssal plain and Iberian continental rise. Although in the northern Atlantic diversity increases basenward as seen in the example above, the work on the sub-CCD assemblages from Iberia demonstrate that species diversity eventually reaches a maximum, probably somewhere on the continental rise. Beyond a certain depth diversity decreases again onto the abyssal plain. This feature is clearly evident in the Paleocene record at Sites 1067, 1069, and 1070, where diversity falls from 36 taxa at the proximal location (Site 1067) to only 15 taxa at the most distal location (Site 1070). The composition of the most abundant taxa along the Iberian transect is given in figure 11.
Figure 11. Taxonomic composition of the most common species along the Iberian Margin – Abyssal Plain transect, ODP Leg 173. Taxa are ranked in order of decreasing dominance, with the most abundant taxon at the top. S = total number of species. Data are from Kuhnt & Urquhart (2001).
In spite of the fact that all sites are currently below 5 km depth and the difference in palaeodepth along this transect is small, a faunal change is readily apparent. The ratio of Glomospira to Recurvoides increases with depth, and diversity decreases in both the Paleocene and Eocene assemblages. The small differences in depth seen in this profile were probably not enough to effect such a faunal change, and to what extent the foraminiferal fauna of the Iberian Margin responded to other environmental factors such as the trophic continuum, bottom currents, etc. remains a matter for speculation.
At this stage it is useful to return to the carbonate dissolution scenario, and ask the question if such situations enhance the niche of "flysch-type", deep water agglutinated foraminifera. Turning the question around, one could start with asking if the rates of carbonate supply, carbonate precipitation and carbonate dissolution control the distribution and preservation of calcareous benthic (and planktonic) foraminifera. Asked this way, the answer is of course yes. Limited carbonate supply, and carbonate corrosive environments hamper production, preservation and burial of calcareous tests, as in deeper water, higher latitudes, restricted basins, and along many continental slopes with high organic production. This fact is in line with the observation that a few strongly etched or largely dissolved calcareous foraminiferal tests are found in Paleogene shales of the North Sea and offshore Canada, which are rich in deep water agglutinated taxa.
However, this does not answer the question if the demise of one group, i.e., calcareous benthics, enhances flourishing of the other, i.e., agglutinating taxa with organic cement. The jury is still out on this question, but the observations of a rich, flysch-type fauna together with a diversified deep water calcareous fauna living on the upper, middle and lower continental slope of the northern Grand Banks (Schafer et al., 1983) suggests that the question is more complex.
A decade after the pioneering work of Schafer et al. (1983), Murray & Alve (1994) showed that diverse Atlantic agglutinated assemblages are living amidst the calcareous foraminiferal fauna on the slope and abyssal plain of the NW European slope facing the Atlantic. The agglutinated component reveals itself well when the calcareous component is gently dissolved in acid. The acid treatment also led to the destruction of agglutinated taxa with a calcareous cement such as Textularia sagittula, Eggerella bradyi, Sigmoilopsis schlumbergeri, Karrerulina bradyi, and Siphotextularia spp. Extremely delicate agglutinated forms like Lagenammina, Nodellum and some of the tubes might not survive fossilisation, and also contribute to taxonomic discrepancies between Recent and fossil deep water foraminiferal assemblages. In likeness to the palaeoecological studies quoted earlier, the authors conclude that post-mortem concentration of agglutinated taxa through the partial or total loss of calcareous taxa by dissolution, is a likely origin of some of the agglutinated assemblages in the fossil record.
We concur with Murray & Alve that process of early diagenetic dissolution can result in residual agglutinated assemblages. This process certainly takes place near the lysocline in the open ocean. However, we believe this is not the only process involved. Importantly, Murray & Alve studied samples from the eastern North Atlantic shelves, a carbonate-rich environment. We ask the question does the modern Atlantic shelf/slope constitute a good modern analogue for the flysch-type assemblages of the Paleogene ocean? Does a reasonable modern analogue for these faunas exist?
The modern South China Sea shares many features in common with the Paleogene Alpine flysch basins. The sea is a deep (>4 km) silled basin with limited deep water exchange with the Pacific Ocean. It is surrounded by tectonically active margins that supply terrigenous detrital material (and volcanic ash) in the form of turbidites. The Philippine side of the South China Sea has virtually no shelf, and ample organic material is swept into the deep basin by downslope movements. The dissolved oxygen content below the thermocline is lower that in the open ocean (around 2 ml/l), while the high nutrient content of deep water results in high levels of productivity during monsoonal upwelling events. The organic carbon content of surficial sediments exceeds 1% at many stations to a depth of 2,000 m, while at deeper stations the TOC is between 0.5 and 0.7%. These ecological factors result in a relatively shallow CCD in the South China Sea, and noncalcareous sediments with rich agglutinated foraminiferal faunas can be cored within sight of Mt. Pinatubo volcano. The distribution of modern agglutinated foraminifera in this basin was documented by Hess (1998).
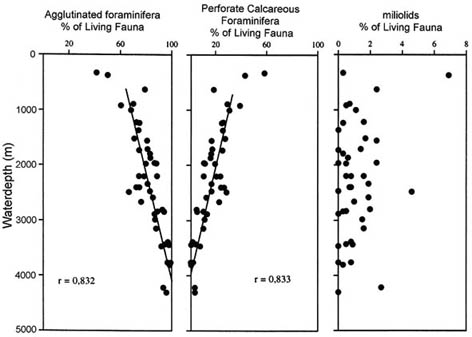
Agglutinated foraminifera comprise over 60% of the living fauna below a water depth of ca. 500m, and this percentage rises steadily to values of 96 - 98 % at a depth of ca. 3,800 m (Fig. 12). The benthic foraminiferal diversity is high, with 60 to 80 species/100 cm2. In this silled basin, most species of agglutinated foraminifera have surprisingly wide bathymetric ranges, occurring from ca. 600 to ca. 4300m depth. However, the faunal density and relative proportions of certain species do display depth-related trends. The upper to middle bathyal assemblages are dominated by tubular forms (Rhizammina and Saccorhiza) trochamminids, and large specimens of Reophax. A faunal break is seen at ca. 2000 m, below which depth abundance and diversity begins to decline. Interestingly, in the data set of Hess (1998), there are no clear depth-related trends among the agglutinated foraminiferal morphogroups. As expected, Morphogroup A (tubular forms) displays a weak inverse correlation with Morphogroup C (deep infauna), but none of the groups shows any strong bathymetrical preference. Nevertheless, several species, though widely distributed, do display higher relative proportions at greater depths, including Saccammina sphaerica, Subreophax adunca, Ammobaculites agglutinans, Haplophragmoides rotulatus, Eratidus foliaceus, Recurvoides contortus, and Karrerulina apicularis. The wide bathymetrical ranges of DWAF in the South China Sea probably result form the lack of deep connections with the Pacific Ocean resulting in a homogeneous deep water mass. Additionally some of the truly abyssal Pacific taxa are likely absent.
Figure 12. Percentages of Rose Bengal stained agglutinated, calcareous benthic, and miliolid foraminifera in boxcore samples collected in the South China Sea (after Hess, 1998).
In conclusion, we may state that flysch-type agglutinated assemblages comprised of taxa with organic cement survive and flourish on low-energy, fine-grained and organic-rich terrigenous substrates that formed under stratified water masses, with a moderate to high Corg and lower CaCO3 productivity. Such "favourable" ecological conditions frequently occur in small, silled basins (such as the South China Sea) with temporal and spatial restriction of oxygen due to the development of oxygen minimum zones in mid water settings. Excess carbon over carbonate production quickly leads to corrosion of foraminiferal tests made of carbonate. Hence the importance of agglutinated foraminifera in the stratigraphy and palaeobathymetry of petroleum basins. Such favourable sedimentary conditions were present during the tectonically active stage of basin formation in the North Atlantic and western Tethyan "flysch" basins. The diversity of species and the proportions of the different morphogroups in DWAF assemblages appears to be a function of the trophic continuum, and in particular the organic carbon flux (Preece et al., 1999). In this regard, the presence of gentle deep-sea currents probably also plays a positive role, especially with regards to the suspension feeding tubular morphogroup. Species diversity is negatively correlated with organic carbon flux, with larger numbers of agglutinated species occurring towards the oligotrophic end of the trophic spectrum (Altenbach et al., 2000). Experience from the high-latitude sites tells us that DWAF begin to disappear when productivity reaches values that are too high, or when sediments become dominated by biosiliceous components. In the latter case, the DWAF appear to be ecologically excluded.
|